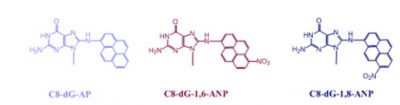
One of the objectives of our research is to understand the biological effects of the DNA adducts formed by nitroaromatic compounds that are suspected to be carcinogenic and mutagenic. Research by us and others has established that mutagenicity of the bulky DNA adducts such as the adducts formed by nitropyrenes depends on a series of factors: (i) structure and conformation of the lesion, (ii) DNA sequence context, (iii) the type of cell in which it is replicated, (iv) whether or not the cells were induced for certain functions (e.g., SOS), and (v) the repair status of the cell. The same adduct may induce different types and frequencies of mutations in different DNA sites and also in different cell lines. It is now believed that the change in mutagenic profile stems from the identity of the DNA polymerase that bypasses the lesion. Our objectives are to identify the DNA polymerase(s) responsible for the observed mutational outcome in a specific cell line and understand the mechanism of their translesion synthesis. Another goal is to determine the orders of mutagenicity, genotoxicity, and efficiency of repair for a group of bulky adducts formed by the nitroaromatic compounds. We have chosen to study the nitropyrenes because 1-nitropyrene is the most abundant nitroarene that pollutes our environment, whereas the dinitropyrenes being much more potent carcinogens/mutagens, provide one the opportunity to investigate structure-activity relationships. 3-Nitrobenzanthrone, a more recently discovered pollutant and a potent carcinogen, is considered a hazard for the general population. It also forms C8 and N2 2′-deoxyguanosine adducts, which provides us another structural dimension to compare with the nitropyrene adducts.
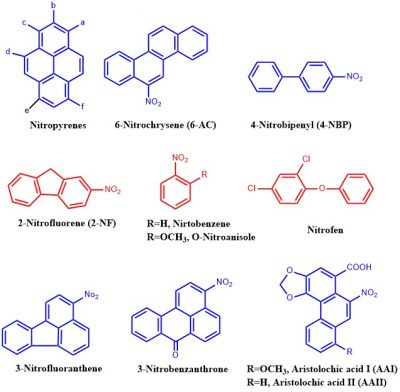
Structures of some of the common nitroaromatic compounds that are likely to be carcinogenic to humans are shown here:
Adducts or lesions in DNA by the nitroaromatic compounds are responsible for the critical biological endpoint. For example, cellular effects of nitropyrenes are initiated by the major DNA adducts shown below:
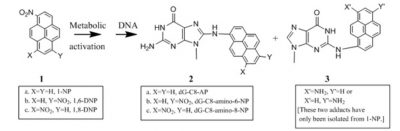
Nitropyrene-derived DNA adducts are mutagenic:
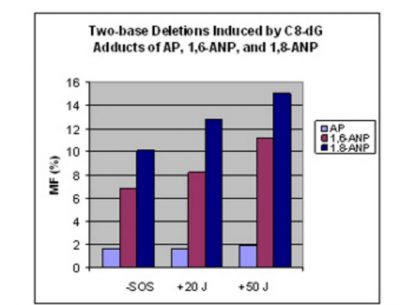
Two-base deletion frequency in (CpG)3 sequence in Escherichia coli varies with the structure of the adduct: