Ionizing radiation causes numerous DNA damages, including base modifications, strand breaks, protein-DNA cross-links, and abasic sites. In irradiated, aerated aqueous solutions hydroxyl radical, which is also generated via normal cellular oxidation, is believed to be the major reactive species. As a result, the DNA damages induced by ionizing radiation are similar to oxidative stress, and these lesions have been implicated in aging and cancer. Many studies indicate significant levels of oxidative lesions in untreated cells (in excess of 100,000 lesions per human cell).
Hydroxyl radical-induced DNA damage is complex, leading to a multitude of modifications of the DNA bases and the deoxyribose sugar moiety. Some of these lesions, such as 8-oxoguanine, have been extensively studied.
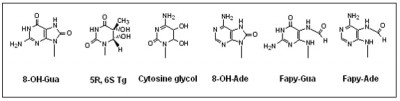
However, there are also complex DNA damages (such as tandem DNA lesions) that are little studied. We have initiated studies of these complex lesions.
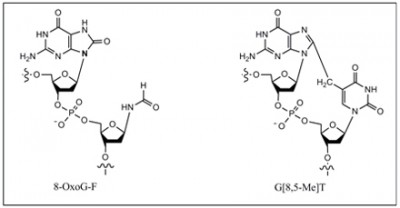
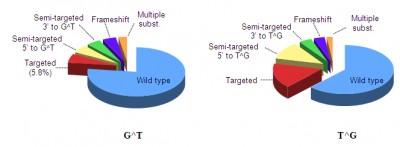
G[8,5-Me]T or G^T is mutagenic in E. coli
G[8,5-Me]T (or GˆT) and T[5-Me, 8]G (or TˆG) are mutagenic in human embryonic kidney (293T) cells and induces a variety of mutations:
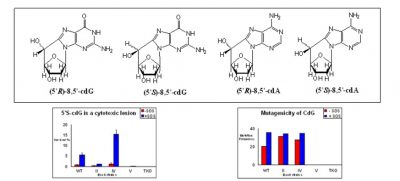
 8,5′-Cyclopurine-2′-deoxynucleosides, another type of tandem lesion in DNA, are both genotoxic and mutagenic:
8,5′-Cyclopurine-2′-deoxynucleosides, another type of tandem lesion in DNA, are both genotoxic and mutagenic:
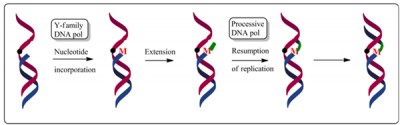
Recent studies established that specialized DNA polymerases that belong to the “Y-family” of DNA polymerases carry out translesion synthesis of DNA lesions, when a processive DNA polymerase is blocked. A more flexible binding site of these enzymes accommodates the bulky or distorting lesions in non-Watson-Crick base pairs. These DNA polymerases lack the 3’→5′ proofreading exonuclease activity, and unlike the normal replicative DNA polymerases that are blocked by these lesions, the Y-family DNA polymerases can bypass the DNA adducts. The following figure shows a simplified scheme as to how a replication blocking lesion may be bypassed by a specialized DNA polymerase that continues synthesis of few additional bases, after which the processive DNA polymerase resumes synthesis.